
Mechanism of Nitration
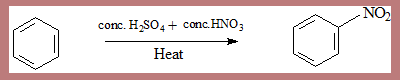
- Substitution of a nitro group into the aromatic ring is nitration.
- It is an aromatic substitution reaction.
- It is an electrophilic substitution reaction.
- Substitution takes place involving an electrophile.
- Similar reactions are: Sulphonation, Halogenation, Alkylation and Acylation.
The mechanism
Generation of electrophile

Step I Formation of σ complex
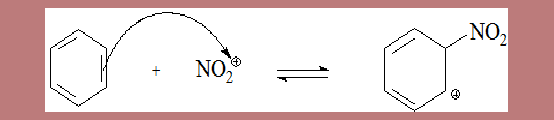
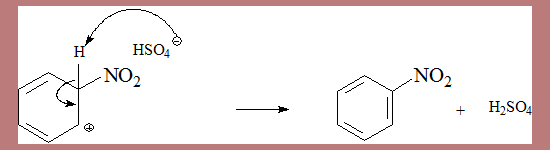
Step II Re-aromatisation
Brief description
- Interaction of sulphuric and nitric acids results in the electrophile nitronium ion NO2+.
- The Pi-electrons of benzene ring attack the electrophile, forming a σ complex.
- Deprotonation of the σ complex, by the base yields the product.
- The σ complex is also known as wheland intermediate or arenium ion. (Advanced organic Chemistry 4th edn page 502, Jerry March )
- It is a two step process. (generation of electrophile is not a step of the mechanism. WHY?)
- The first step is slow and reversible. It is slow because bond breaking is involved; hence there is an activation energy barrier.
- A stable aromatic compound is becoming a fairly unstable product. It is reversible because the products in the step can easily recombine to go back to the reactants (very little activation energy barrier)
- The second is the fast step (formation of a highly stable product from an unstable reactant, rearomatisation)
- The base HSO4- deprotonates the complex in the second step.
- H2SO4 donates a proton to HNO3 hence it is an acid and HNO3 is a base in comparison in this specific reaction.
Copyrights: 2005 www.chemvista.org All Rights Reserved